Energy metabolism is essential to all living organisms. To mediate energy transfer, nature uses ATP and pyridine nucleotide coenzymes such as reduced nicotinamide adenosine dinucleotide (NADH) as energy carrier (EC). Because natural EC link multiple energy transfer modules and biological processes, it is intrinsically challenging to control energy transfer at the EC level.
To establish selective control over cellular energy transfer, Prof. ZHAO Zongbao and his co-workers at Dalian Institute of Chemical Physics, CAS, proposed to implement non-natural cofactor as EC. In an early study, ZHAO’s group chemically synthesized nicotinamide cytosine dinucleotide (NCD), and engineered some redox enzymes to use NCD (J. Am. Chem. Soc., 2011, 133, 20857). It was found that those engineered enzymes could function similarly to their natural counterparts in terms of catalytic efficiency and substrate/product selectivity.
In the current study, efforts have led to generating phosphite dehydrogenase mutant (Pdh*) to favor NCD and realized efficient production of NCDH at the expense of phosphite (ACS Catalysis, DOI: 10.1021/acscatal.6b03579). By using in vitro multi-enzyme systems, more experiments showed that NCDH-linked subsystem functioned independent of natural cofactor-linked redox system.
ZHAO’s group then devised Escherichia coli strain that overexpressed Pdh*, NCD-linked malic enzyme (Mae*) and nucleotide transporter (Ndt). It was found that, cells took up NCD and used phosphite as the electron source to generate NCDH that drove reductive carboxylation of pyruvate for improved malate production from glucose. Thus, it provides an opportunity to establish an orthogonal energy-transfer system for engineering cell factories and may be used to set an additional layer of a control mechanism for life.
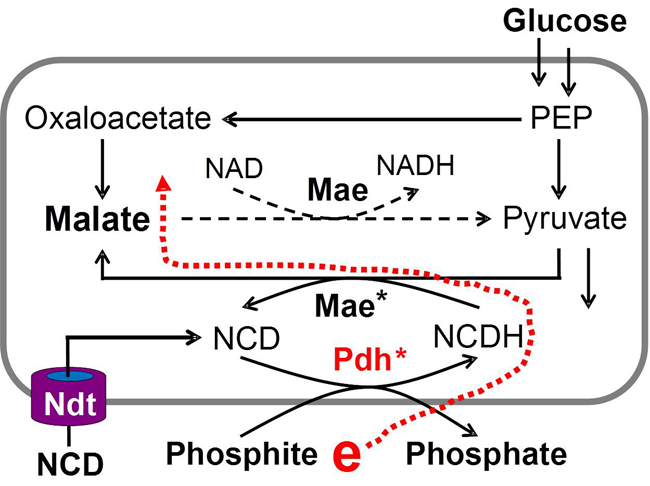
Figure. E. coli WL005 harboring the circuit Pdh-Mae-NCD and expressing the transporter Ndt (Image by LIU Wujun)
The research work was financially supported by Minister of Science and Technology of China, National Natural Foundation of China and State Key Laboratory of Catalysis.(Text and Image by LIU Wujun)